The title of this blog might make you think it's as simple as teaching me to “sit,” but thermal imaging is actually pretty complex. On the bright side, it lets us see things we normally can't, like hidden threats in the wild, misbehaving machines, and a dog like me, just trying to do my thing.
Objects Are Constantly Glowing
Everything is constantly giving off light in the form of waves of electromagnetic energy. Our eyes can only see visible light, with a wavelength ranging from 400 nm (blue light) to 780 nm (red light), but heat signatures are emitted in the infrared, which is invisible to humans (although a dog like me can sense it with my nose). Infrared light has wavelengths ranging from 800 nm to about 14 μm, as noted in this blog on microscopy written by humans.
The wavelength (λ) and intensity (E) of the light emitted from an object depends on its temperature (T) and some universal constants, leashed together by Planck’s law.
Equation 1. Energy irradiated as a function of wavelength
Figure 1. Planck’s law, 0-100 °C, 0-14 μm
Planck’s law shows that at the lower temperatures that most animals or organic materials can tolerate, there's no radiation in the visible range, see Figure 1. That's why we can't see the heat from objects with our eyes. But a thermal imaging camera can see these wavelengths for us!
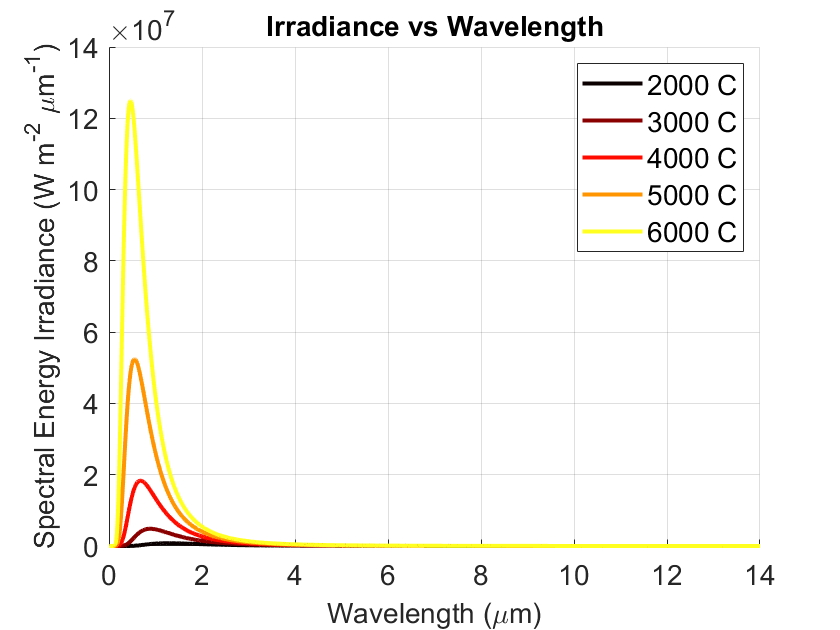
Figure 2. Planck’s law, 2000-6000 °C, 0-14 μm
Figure 3. Planck’s law, 2000-6000 °C, 0-1 μm
When temperatures get really high, the radiated light shifts towards the visible range, see Figures 2 and 3. That's why we can see a metal or flame that is glowing “red hot” or even hotter - blue!
Most thermal imaging cameras are simply sensors that measure an average radiation within the 8 - 14 μm range. With a bunch of these sensors tiled together, the camera can resolve an image. From Figure 4, we can see how the higher the temperature, the more radiation and the brighter the object will appear in the camera. Maybe this still sounds simple?
Figure 4. Planck’s law, 0-100 °C, 8-14 μm
Real Objects Have Variable Emissivity
Here's where it gets tricky. Planck’s law applies to something called a “perfect black body” that emits 100% of its thermal energy. But real materials have a lower emissivity than a perfect black body, and the image seen by the camera will be the product of the object’s emissivity (ε) and the perfect black body emission at the object’s temperature:
Some examples of common objects and their emissivity’s are shown in Table 1. Most organic materials like plastics, plant fibers, and dogs have high emissivity, while inorganics can range wildly, with metals having very low emissivity. To make matters worse, surface textures can affect emissivity, as ruff surfaces may have higher emissivity than smooth ones.
Table 1. Objects and their emissivities
Now we see why this is hard! A hot object with a low emissivity, like metal, may look to the thermal camera exactly the same as a cooler object with a high emissivity, like plastic. So that camera can only accurately measure the temperature of an object if you already know the object’s emissivity.
Figure 5 shows a box painted gray, with strips of aluminum foil tape aligned diagonally. The entire box including the gray paint and foil tape is the same temperature - about 30 °C (86 °F). However, the painted strips look warm, whereas the metal strips look cold. This is because the metal strips are actually reflecting the room around them, which is closer to 20 °C (68 °F), while not emitting much of their own heat at all. In Figure 5, the metal strips are reflecting the cool, 20 °C (68 °F), room. But in Figure 6, the metal strips are reflecting a warm 37 °C (98 °F) human in the room. You can see his warm (albeit distorted) face and waving hand. This is really complex - woof!
Figure 5. A photo of a box and an image of the box in IR
Figure 6. A photo of a box and an image of the box in IR
My Time to Shine!
Given these basics, let’s take a look at an image we all care about. Figure 7 shows a picture of me trying to have a drink in peace, taken with a thermal imaging camera. My body temperature is a healthy 103 °F, but when you add in the insulating fur I’ve yet to shed on the couch and the mess of water you’ll be soaking up with your socks later, my thermal image becomes a work of art!
Figure 7. Visible and infrared portraits of the author
What does this mean for SMS?
At SMS, where I oversee security, researchers are manipulating surface emissivity through techniques like micro and nanoscale texturing. Additionally, they are creating plasmonic metamaterials with unique spectral signatures by patterning metal at the subwavelength level. This allows them to give natural surfaces unnatural thermal signatures, and vice versa.
Thanks for making it this far - here are some videos of me making a mess of my water bowl and taking our VP of R&D for a walk. What else can you see??