Many plants and insects exhibit self-cleaning properties as a result of the hydrophobic nature of their leaves and wings. Instead of spreading out on these surfaces, water beads up into a tight ball that minimizes its contact with the surface. This “superhydrophobic” interaction is commonly referred to as the lotus effect because it was first studied in lotus plants. The top of the lotus leaf consists of hierarchical micro- and nanofeatures that enhance the hydrophobicity of the waxy leaf. To demonstrate, I grew a lotus leaf and imaged the surface and its interactions with water droplets, seen in Figure 1. If this natural phenomenon can be harnessed, for example in food safe non-stick coatings, anti-icing surfaces for airplanes, or oil-repellent surfaces for camera lenses, it can improve our everyday lives.
Figure 1. SEM images of SMS's lotus leaf and a photo of the leaf diplaying hydrophobicity
But what causes water to show a fear of the surface and bead up in hydrophobic interactions but spread out readily in hydrophilic ones? The answer lies in both the chemistry and texture of the surfaces. Every surface or interface holds energy due to the broken or dangling bonds searching for a chemical mate. The most stable state of the droplet on the surface is the state that minimizes the area of the high-energy interfaces while maximizing the area of the low-energy interfaces.
When considering a droplet on a solid surface, there are three interface energies to consider: the liquid-vapor, solid-liquid, and solid-vapor interface energies. A simple way to evaluate the interaction between these three values on an untextured surface is Young’s contact angle - the angle of the liquid-vapor interface measured through the droplet. Contact-angle measurements are often used as a measure of the hydrophobicity of a planar surface due to challenges in measuring the surface interactions.
Figure 2. The states of a water droplet on a surface and the associated contact angles.
The solid-liquid interfacial energy ( γsl) can be used to predict changes in Young's contact angle, assuming the solid and vapor remain the same. A high γsl leads to a higher contact angle because the liquid and solid don’t want to be in contact with each other - this causes the water to attempt to find stability elsewhere by creating a bead of water (Figure 2a,b). The inverse is also true - a low γsl leads to a low contact angle as the interface is stable and the liquid and solid want to remain in contact with each other (Figure 2c,d).
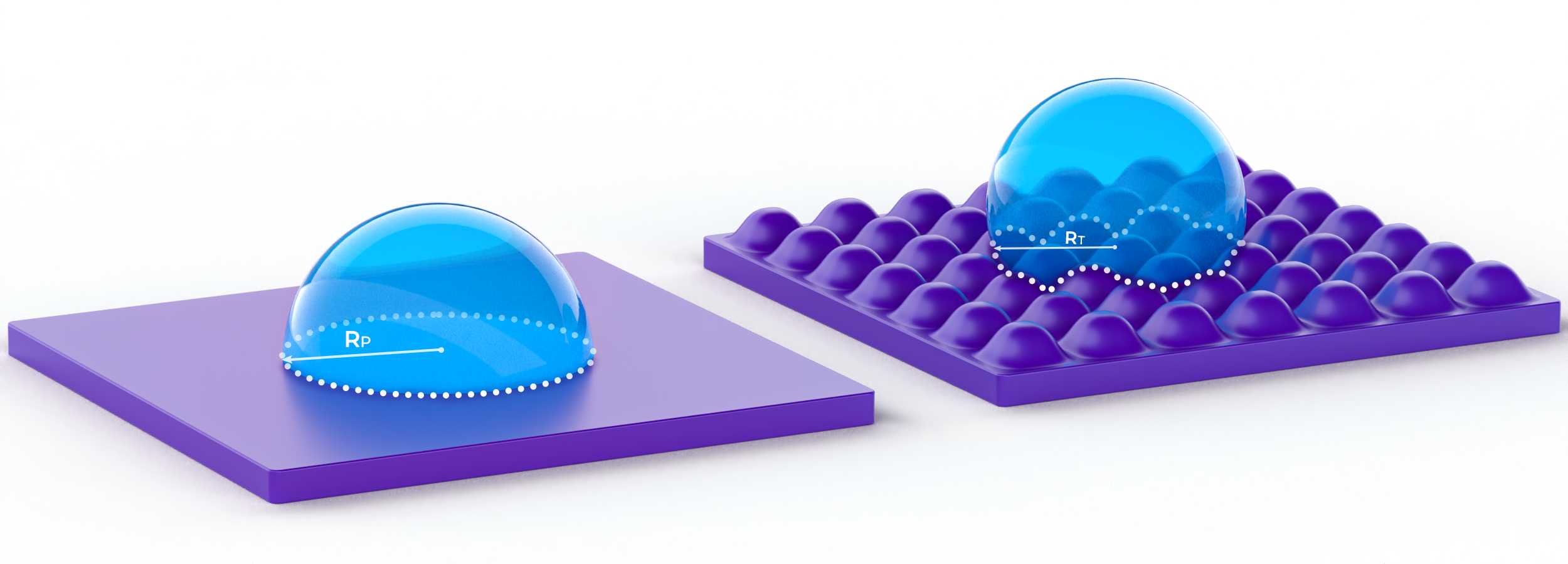
Texturing a surface can enhance the existing interaction between a liquid and a surface - in fact, it is currently only possible to achieve superhydrophobicity with a textured surface. This enhancement occurs because applying a texture increases the surface area and therefore increases the intensity of surface energy on the solid, changing the balance between the three interfaces. Assuming the interaction started hydrophobic, the water droplet will continue wanting to have minimal contact with the solid. The radius of the droplet will shrink from RP on the planar surface to RT on the textured surface, resulting in the enhanced hydrophobic interaction seen in Figure 3.
Figure 3. The hydrophobic enhancement between planar and textured surface. The dashed lines surround the solid-liquid interface of the droplets.
There are two possible states for a droplet on textured surfaces, the Wenzel state and the Cassie-Baxter state (Figure 4). In both states the interaction can be measured via the apparent contact angle (θ*). In the Wenzel state, the droplet has fully wetted the texture; a hydrophobic surface will become more hydrophobic and a hydrophilic surface will become more hydrophilic. In the Cassie-Baxter state, the texture is partially wetted with air trapped between the bottom of the texture and the droplet. Textures with drops in the Cassie-Baxter state always increase the hydrophobicity of hydrophobic surfaces, but may not increase the hydrophilicity of hydrophilic surfaces.
Figure 4. A water droplet on a planar surface, a droplet in the Wenzel State, and a droplet in the Cassie-Baxter state. Note that the droplet reaches the bottom of the texture in the Wenzel state, but air is trapped below the droplet in the Cassie-Baxter state.
Figure 5. Contact angle of water on a patterned vs smooth surface (silane treated polycarbonate).
The hydrophobic surfaces found in nature can be replicated and engineered for individual applications ranging from liquid-repellent fabrics to drag-reduced surfaces. For example, the Kota Research Group at NCSU has worked to create omniphobic fabrics that can be used as chemical shields against almost all contacting liquids. While SMS has not optimized a surface for hydrophobicity, an improvement in the apparent contact angle has been achieved in small experiments with existing replicas (Figure 5).